The group V elements include: Nitrogen, Phosphorus, Arsenic, Antimony and Bismuth. There are more differences than similarities between the elements in this group.
Table of Contents
- Nitrogen is a diatomic gas and does not exhibit allotropy. Phosphorus is a typical non-metal and exhibits allotropy. Arsenic and Antimony are metalloid; while bismuth is a metal.
- Nitrogen is a colourless gas. Phosphorus exists in variety of allotropic forms like white and red phosphorus. Arsenic exists as a dull grey metallic solid. Antimony and bismuth is silvery-white solid.
- Nitrogen is the most electronegative element in the group. It combines with metals and non-metals to form a variety of compounds.
ELECTRONIC CONFIGURATION
The electronic configuration of the group V elements is as follows:
Nitrogen = 7: 1s2 2s2 2p3
Phosphorus = 15: 1s2 2s2 2p6 3s2 3p3
Arsenic = 33: 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p3
Antimony = 51: 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s2 5p3
NITROGEN
Nitrogen occurs chiefly as a free element in the air. It makes up 78% by volume of the atmosphere. It also exists in combined form in many compounds e.g ammonia, urea, proteins etc.
LABORATORY PREPARATION
From Air
It can be obtained from air by passing air through caustic soda to remove CO2 and
overheated copper turnings to remove O2 respectively. The nitrogen obtained is not pure, it contains 1% by volume of rare gases and it is denser than air.
Pure nitrogen is obtained in the laboratory by chemical method. The following chemicals are used to prepare nitrogen.
- Thermal decomposition of ammonium dioxonitrate (III)
- NaNO2(aq) + NH4Cl(aq) → NH4NO2(aq) +NaCl(aq)
- NH4NO2(aq) → 2H2O(l) + N2(g)
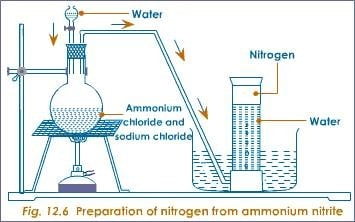
- Thermal decomposition of ammonium heptaoxodichromate (VI)
(NH4)2Cr2O7(s) → Cr2O3(s) + 4H2O(l) + N2(g)
- Oxidation of ammonia by hot Copper (II) oxide
2NH3(g) + 3CuO(s) → 3Cu(s) + 3H2O(g) + N2(g)
- Reduction of dinitrogen (I) oxide by red-hot copper.
N2O(g) + Cu(s) → CuO(s) + N2(g)
INDUSTRIAL PREPARATION
Industrially, nitrogen is obtained by fractional distillation of liquid air.
EVALUATION
- Write the electronic configuration of nitrogen and phosphorus.
- Using balanced equations, state TWO methods of preparing nitrogen in the laboratory.
PHYSICAL PROPERTIES
- It is colourless, odourless and tasteless
- Pure nitrogen is lighter than air.
- Slightly soluble in water
- Melting point – 2100C and boiling point is -1960C
CHEMICAL PROPERTIES
- It reacts with very electropositive metals to form nitrides
3Mg(s) + N2(g) → Mg3N2(s)
N2(g) + 3H2(g) → 3NH3(g)
N2(g) + 2O2(g) → 2N2O(g)
USES
- It is used industrially to manufacture ammonia.
- Liquid nitrogen is used as a cooling agent.
- It is used as preservative in packaged foods to prevent rancidity.
EVALUATION
- State TWO physical properties and TWO chemical properties of nitrogen.
- Mention THREE uses of nitrogen.
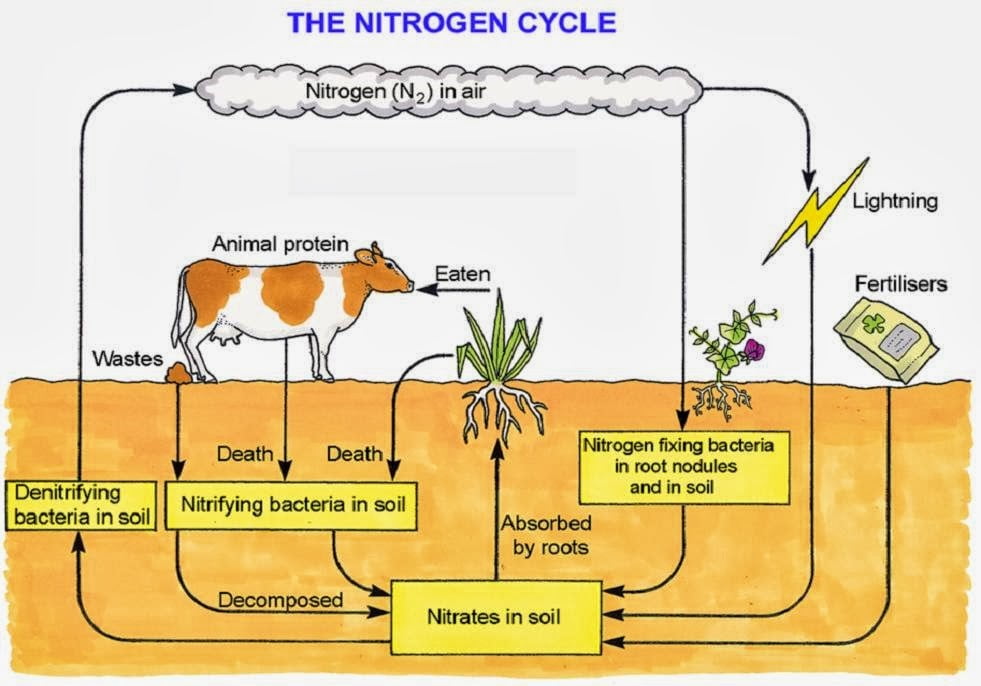
The stages in which atmospheric nitrogen is converted into soil nitrogen and back to free nitrogen occurs in the following ways:
- Oxidation of atmospheric nitrogen: During lightning and thunderstorm, there is electrical discharge in the atmosphere which cause atmospheric nitrogen and oxygen combine to form oxides of nitrogen which can dissolve in rain water as dioxonitrate (III) and trioxonitrate (V) acids. These acids go into the soil and react with mineral salts in the soil to form trioxonitrate (V) salts which is absorbed by plants.
N2(g) + O2(g) 2NO(g)
2NO(g) + O2(g) 2NO2(g)
4NO(g) + O2(g) + 2H2O(l) 4HNO2(aq)
4NO(g) + O2(g) + 2H2O(l) 4HNO3(aq)
- Action of nitrogen-fixing bacteria: Soil micro-organisms like Rhizobium living in root nodules of leguminous plants are able to convert atmospheric nitrogen into organic nitrogenous compounds which are used directly by the host plants. These compounds are released into the soil when these plants die. Other free living micro-organisms in the soil like Azotobacter and Clostridium are also able to convert atmospheric nitrogen directly into trioxonitrate (V) which is absorbed by plants. These processes are known as nitrogen fixation.
- Decay of organic matter: When plants and animal die, putrefying bacteria and fungi in the soil converts organic nitrogenous compounds to ammonia. Nitrifying bacteria like Nitrosomonas and Nitrobacter convert the ammonia to trioxonitrate (V) which can again be absorbed by plants. These processes are known as nitrification.
- Denitrification: Trioxonitrate (V) salt in the soil can be converted to gaseous nitrogen by denitrifying bacteria. The process is known as Denitrification. The nitrogen so formed escapes into the atmosphere, where it becomes atmospheric nitrogen. Denitrifying bacteria therefore reduces the quantity of trioxonitrate (V) in the soil.
GENERAL EVALUATION/REVISION
- Using balanced equation ONLY, show how nitrogen can be prepared in the laboratory.
- Outline the stages involved in the nitrogen cycle.
- Write the electronic configuration of N3- and P5-
- Balance the following redox equation: I– + MnO4– → IO3– + MnO2 in basic medium
- State the effect of the following on the equilibrium position of the reaction below: 3Fe(s) + 4H2O(g) Fe3O4(s) + 4H2(g) ∆H = +ve
- Increase in temperature
- Increase in pressure
- Using iron filings instead of iron rod.
WEEKEND ASSIGNMENT
SECTION A: Write the correct option ONLY
- In which group of the periodic table is nitrogen found? (a) 2 (b) 5 (c) 7
(d) 6
- The boiling point of nitrogen in 0C is (a) -183 (b) -196 (c) 200 (d) 240
- The percentage of nitrogen in air is (a) 78 (b) 75 (c) 71 (d) 67
- The following are uses of nitrogen except a. as a cooling agent b. to prevent rancidity c. in the manufacture of fertilizers d. in laundry.
- The atomicity of nitrogen is (a) 1(b) 2 (c) 3 (d) 4
SECTION B
- Briefly describe the preparation of nitrogen from air in the laboratory.
- State three physical properties and two chemical properties of nitrogen.
See also
CHEMICAL EQUILIBRIUM | STATIC, DYNAMIC, REVERSIBLE REACTION, PROPERTIES, FACTORS & EFFECTS
